Despite the illegal destruction of most of the structural steel and other debris in the months after 9/11, a sizeable body of forensic evidence has been developed over the years by government investigators and independent researchers. Much of the available evidence indicates the occurrence of high-temperature thermitic reactions before, during, and after the destruction of the towers.
NFPA 921: Guide for Fire and Explosion Investigations, which serves as the national guide for fire and explosion investigations in the United States, advises that “All available fuel sources should be considered and eliminated until one fuel can be identified as meeting all the physical damage criteria as well as any other significant data.” [Emphasis added.] On the potential use of exotic accelerants, including thermite, NFPA 921 further advises: “Indicators of exotic accelerants include...melted steel or concrete.”
The National Institute of Standards and Technology (NIST), however, did not follow NFPA 921 in its investigation of the World Trade Center destruction. Instead, it handled the evidence of high-temperature thermitic reactions in much the same way it handled the evidence regarding the structural behavior of the buildings: either denying it, ignoring it, or providing speculative explanations not based upon scientific analysis.
Molten Metal Pouring out of WTC 2
Just before 9:52 AM, molten metal began pouring out of WTC 2 near the northeast corner of the 80th floor and continued to flow with increasing intensity until the collapse at 9:59 AM. NIST provided ample documentation of the pouring molten metal, which it described and hypothesized as follows:

 Molten metal pouring out of WTC 2.
Molten metal pouring out of WTC 2.
“Just over a second [after 9:51:51 AM], a bright spot appeared at the top of one window...and a glowing liquid began to pour from this location.... The composition of the flowing material can only be the subject of speculation, but its behavior suggests it could have been molten aluminum.... The Aluminum Association Handbook...lists the melting point ranges for the alloys [comprising the Boeing 767 structure] as roughly 500°C to 638°C and 475°C to 635°C.... These temperatures are well below those characteristic of fully developed fires (c. 1,000°C)....” 1
But, as Dr. Steven Jones writes in “Why Indeed Did the WTC Buildings Completely Collapse?” this claim is untenable due to the color of the molten metal:
“Is the falling molten metal from WTC Tower 2...more likely molten iron from a thermite reaction OR pouring molten aluminum?
“The yellow color implies a molten metal temperature of approximately 1,000°C, evidently above that which the dark-smoke hydrocarbon fires in the Towers could produce.... Also, the fact that the liquid metal retains an orange hue as it nears the ground...further rules out aluminum....
“We also noted [in our experiments] that...the falling aluminum displayed a silvery-gray color, adding significantly to the evidence that the yellow-white molten metal flowing out from the South Tower shortly before its collapse was NOT molten aluminum.” 2
 A thermite reaction.
A thermite reaction.
In its FAQs posted in August 2006, almost a year after the release of its final report, NIST attempted to address the criticism that molten aluminum would have a silvery appearance:
“Pure liquid aluminum would be expected to appear silvery. However, the molten metal was very likely mixed with large amounts of hot, partially burned, solid organic materials... which can display an orange glow, much like logs burning in a fireplace.” 3
 Molten aluminum.
Molten aluminum.
While NIST did not test its hypothesis — merely asserting that it was “very likely” — Dr. Jones did:
“NIST states the hypothesis that flowing aluminum with partially burned organic materials mixed in, “can display an orange glow.” But will it really do this? I decided to do an experiment to find out.... Of course, we saw a few burning embers, but this did not alter the silvery appearance of the flowing, falling aluminum....
“In the videos of the molten metal falling from WTC 2 just prior to its collapse, the falling liquid appears consistently orange, not just orange in spots and certainly not silvery. We conclude from all of these studies that the falling metal which poured out of WTC 2 is NOT aluminum.” 4
More than a decade later, NIST still has not conducted its own experiments to verify its hypothesis, nor has it revised its FAQs to account for the results of Dr. Jones’ experiments.
Molten Metal in the Debris
Not only was molten metal seen pouring out of WTC 2, dozens of eyewitnesses observed it in the debris of all three buildings. A small selection is presented below (a comprehensive list of all eyewitness accounts to molten metal can be found in the article “Witnesses of Molten Metal at Ground Zero”):
- Leslie Robertson, a lead engineer in the design of WTC 1 and WTC 2, told an audience: “We were down at the B-1 level and one of the firefighters said, ‘I think you’d be interested in this.’ And they pulled up a big block of concrete, and there was like a little river of steel flowing.” 5
- FDNY Captain Philip Ruvolo recalled with other firefighters seated next to him: “You’d get down below and you’d see molten steel, molten steel, running down the channel rails, like you’re in a foundry, like lava.” Other firefighters chimed in: “Like lava.” “Like lava from a volcano.” 6
- Ken Holden, the Commissioner of the NYC Department of Design and Construction, testified before the 9/11 Commission: “Underground it was still so hot that molten metal dripped down the sides of the wall from Building 6.” 7

According to NIST, the highest temperature reached by the fires was 1,100°C. Yet structural steel does not begin to melt until about 1,482°C (2,700°F). How then did NIST explain the evidence of molten metal?
NIST’s first approach was to omit the evidence of molten metal from its final report. Then, in its August 2006 FAQs, it addressed that evidence with the following question and answer.
13. Why did the NIST investigation not consider reports of molten steel in the wreckage from the WTC towers?
NIST investigators...found no evidence that would support the melting of steel in a jet-fuel ignited fire in the towers prior to collapse. The condition of the steel in the wreckage of the WTC towers (i.e., whether it was in a molten state or not) was irrelevant to the investigation of the collapse since it does not provide any conclusive information on the condition of the steel when the WTC towers were standing....
Under certain circumstances it is conceivable for some of the steel in the wreckage to have melted after the buildings collapsed. Any molten steel in the wreckage was more likely due to the high temperature resulting from long exposure to combustion within the pile than to short exposure to fires or explosions while the buildings were standing.
Each claim in NIST’s answer is demonstrably unscientific:
- In the first sentence, NIST assumes that the only possible cause of “melting steel” would have been “the jet-fuel ignited fire in the towers,” which is an implausible hypothesis on its face.
- NIST’s next claim — “The condition of the steel in the wreckage...was irrelevant to the investigation...since it does not provide any conclusive information on the condition of the steel when the WTC towers were standing” — flies in the face of forensic investigation principles. Recall NFPA 921, which explicitly advises, “Indicators of exotic accelerants include...melted steel or concrete.” Furthermore, in science, evidence is not ignored on the basis that it is not conclusive by itself. NIST’s claim is yet more perplexing because molten metal was observed pouring out of WTC 2 — “when the WTC towers were standing” — as NIST documented extensively.
- NIST’s next claim is simply false. It is impossible for a diffuse hydrocarbon fire to reach temperatures close to the 1,482°C (2,700°F) required to melt steel, particularly in an oxygen-starved debris pile.
- Finally, with the expression “Any molten steel in the wreckage,” NIST neither confirmed nor denied the existence of molten metal. In an investigation that followed NFPA 921, NIST would have sought to establish whether molten metal was present and, if so, what its source was.
However, outright denial would be the approach used by NIST investigator John Gross. In a talk at the University of Texas in October 2006, he responded to a question about the presence of molten metal with the following answer:
“First of all, let’s go back to your basic premise that there was a pool of molten steel. I know of absolutely nobody, no eyewitness who has said so, nobody who’s produced it. I was on the site. I was on the steel yards. So I don’t know that that’s so. Steel melts at around 2,600°F. I think it’s probably pretty difficult to get that kind of temperatures in a fire.” 8
Iron Spherules and Other Particles in the WTC Dust
Three scientific studies have documented evidence in the WTC dust that indicates extremely high temperatures during the destruction of WTC 1 and WTC 2 — and possibly WTC 7.
The RJ Lee Report
Released in May 2004, the RJ Lee report titled WTC Dust Signature identified “[s]pherical iron and spherical or vesicular silicate particles that result from exposure to high temperature” in the dust.
An earlier 2003 version of RJ Lee’s report observed:
“Various metals (most notably iron and lead) were melted during the WTC event, producing spherical metallic particles. Exposure of phases to high heat results in the formation of spherical particles due to surface tension.... Particles of materials that had been modified by exposure to high temperature, such as spherical particles of iron and silicates, are common in the WTC dust...but are not common in normal office dust.”
The 2003 version also reported that while iron particles make up only 0.04 percent of normal building dust, they constituted 5.87 percent of the WTC dust.
Iron does not melt until 1,538°C (2,800°F), which, as discussed above, cannot be reached by diffuse hydrocarbon fires. Still, even higher temperatures than 1,538°C were indicated by another discovery documented in RJ Lee’s report:
“The presence of lead oxide on the surface of mineral wool indicates the existence of extremely high temperatures during the collapse which caused metallic lead to volatilize, oxidize, and finally condense on the surface of the mineral wool.”
The 2003 version also referred to temperatures “at which lead would have undergone vaporization.” For such vaporization to occur, lead would need to have been heated to its boiling point of 1,749°C (3,180°F).
The USGS Report
Released in 2005, a report by the U.S. Geological Survey (USGS) titled Particle Atlas of World Trade Center Dust identified “trace to minor amounts” of “metal or metal oxides” in the WTC dust and presented micrographs of these particles, two of which were labeled “Iron-rich sphere.”
 A scanning electron microscopy image with EDS of an “iron-rich sphere” provided by USGS.
A scanning electron microscopy image with EDS of an “iron-rich sphere” provided by USGS.
Steven Jones et al.
Published by Dr. Steven Jones and seven other scientists in early 2008, the paper “Extremely high temperatures during the World Trade Center destruction” connected the dots between the earlier RJ Lee and USGS reports. It also provided new observations based on analysis of WTC dust samples obtained by Dr. Jones. According to the authors:
“The formation of spherules in the dust implies the generation of materials somehow sprayed into the air so that surface tension draws the molten droplets into near-spherical shapes. The shape is retained as the droplet solidifies in the air.”
In addition to observing spherules of iron and silicates, their study discussed the presence of molybdenum spherules documented by the USGS study but not included in its report. (This additional data from the USGS study was obtained through a FOIA request.) Molybdenum is known for its extremely high melting point of 2,623°C (4,754°F).
Jones’ study also discussed evidence of even higher temperatures contained in the RJ Lee report (quoting from the RJ Lee report):
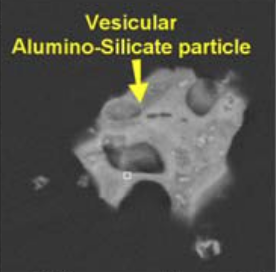 A scanning electron microscopy image with EDS of vesicular alumino-silicate provided by RJ Lee.
A scanning electron microscopy image with EDS of vesicular alumino-silicate provided by RJ Lee.
“Some particles show evidence of being exposed to a conflagration such as spherical metals and silicates, and vesicular particles (round open porous structure having a Swiss cheese appearance as a result of boiling and evaporation).... These transformed materials include: spherical iron particles, spherical and vesicular silicates, and vesicular carbonaceous particles.”
Dr. Jones and his coauthors observed:
“[I]f the “Swiss-cheese appearance” is indeed the result of “boiling and evaporation” of the material as the [RJ Lee] report suggests, we note the boiling temperature for aluminosilicate is approximately 2,760°C.”
They then provided a table summarizing the temperatures needed to account for the various evidence of high temperatures in the World Trade Center destruction, which they contrasted with the much lower maximum temperatures associated with the fires on September 11.
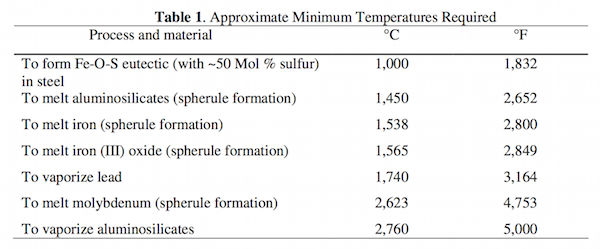 From the paper “Extremely high temperatures during the WTC destruction.”
From the paper “Extremely high temperatures during the WTC destruction.”
The closest NIST has come to acknowledging the evidence of extremely high temperatures in the WTC dust was in an email communication with an independent researcher following the release of NIST’s draft report on WTC 7. NIST replied to the researcher’s inquiry with a single sentence: “The NIST investigative team has not seen a coherent and credible hypothesis for how iron-rich spheres could be related to the collapse of WTC 7.”10
Nano-thermite in the WTC Dust
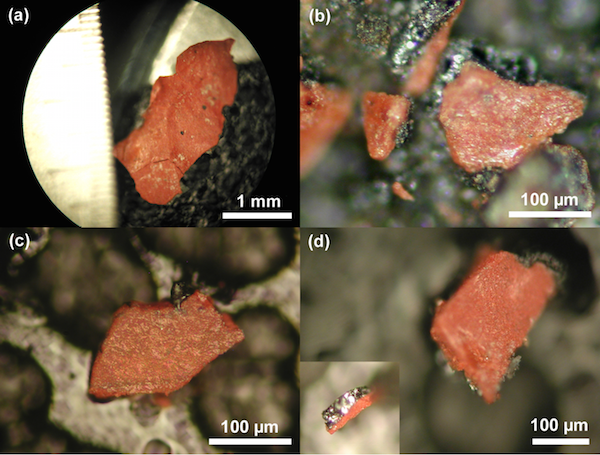 Photomicrographs of red-gray chips from each of the four WTC dust samples. The inset in (d) shows the gray layer of the chips.
Photomicrographs of red-gray chips from each of the four WTC dust samples. The inset in (d) shows the gray layer of the chips.
In April 2009 a group of scientists led by Dr. Niels Harrit, an expert in nano-chemistry who taught chemistry at the University of Copenhagen for over 40 years, published a paper in the Open Chemical Physics Journal titled “Active Thermitic Materials Discovered in Dust from the 9/11 World Trade Center Catastrophe.” 11 This paper, which reported the results of experiments conducted on small red-gray, bi-layered chips found in multiple independent WTC dust samples, concluded that the chips were unreacted nano-thermite, a form of thermite with explosive properties engineered at the nano-level.
According to their analyses, the gray sides of the chips consisted of “high iron and oxygen content including a smaller amount of carbon,” while the red sides had various features indicative of thermite and nano-thermite.
Features Indicative of Thermite
- The chips were composed primarily of “aluminum, iron, oxygen, silicon, and carbon.” The first three elements are suggestive of thermite, which is commonly made by combining aluminum and iron oxide.
- Their red color and magnetic properties were suggestive of iron.
- They all ignited between 415° and 435°C, producing highly energetic reactions.
Features Indicative of Nano-thermite
- The chips’ primary ingredients were ultra-fine grain, seen typically “in particles at the scale of tens to hundreds of nanometers.”
- The ultra-fine ingredients were intimately mixed.
- When a flame was applied to them, it resulted in a “high-speed ejection of a hot particle.”
- They ignited at a much lower temperature — 430°C — than the temperature at which conventional thermite ignites, which is above 900°C.
- Silicon was one of their main ingredients, and it was porous, suggesting the thermitic material was mixed in a sol-gel to form a porous reactive material.
- Their carbon content was significant. The authors noted that this “would be expected for super-thermite formulations in order to produce high gas pressures upon ignition and thus make them explosive.”
 A backscattered electron image of a red-gray chip.
A backscattered electron image of a red-gray chip.
The presence of the above-described substance in the WTC dust strongly suggests that nano-thermite was used in the destruction of WTC 1, WTC 2, and WTC 7.
What other explanations for this substance exist?
The first possibility is that the red-gray chips were in fact paint chips. The researchers explored this possibility — first by soaking the chips in methyl ethyl ketone (a solvent known to dissolve paint chips, which did not succeed in dissolving the red- gray chips), and second by exposing the red-gray chips and known paint chips to a hot flame. The paint chips dissolved into ash, while the red-gray chips did not.
The second possibility is that the WTC dust might somehow have been contaminated with the red-gray chips during the cleanup operation. However, this hypothesis was ruled out on the basis that all four of the dust samples had been collected at times or places that precluded any contamination. One sample was collected about 20 minutes after the collapse of WTC 1. Of the other three samples, two were collected the next day.
With those two possibilities ruled out, no other plausible explanation has been provided — nor has NIST responded to the reported discovery of nano-thermite in the WTC dust.
Therefore, the presence of unreacted nano-thermite in the WTC dust — which is corroborated by other evidence of high-temperature chemical reactions — constitutes compelling evidence that WTC 1, WTC 2, and WTC 7 were destroyed by controlled demolition using nano-thermite and possibly other explosive and incendiary materials.
NIST’s Refusal to Test for Explosives or Thermite Residues
Despite the compelling evidence for high-temperature thermitic reactions examined above, NIST has refused to test for explosives or thermite residues. NIST provides the following question and answer in its FAQs on WTC 1 and WTC 2:
Was the steel tested for explosives or thermite residues?
NIST did not test for residues of these compounds in the steel.... Analysis of the WTC steel for the elements in thermite/thermate would not necessarily have been conclusive. The metal compounds also would have been present in the construction materials making up the WTC towers, and sulfur is present in the gypsum wall- board that was prevalent in the interior partitions.
But, to reiterate the point mentioned above, evidence is not ignored in science just because it is not conclusive. In fact, NIST conducted many tests during the course of its investigation that were not conclusive. Given the evidence examined in this chapter, some of which had already been discussed widely during NIST’s investigation, NIST had every reason to conduct very simple lab tests for explosives and thermite residues, regardless of whether or not such testing would have been conclusive.
Moreover, NIST’s answer actually implies that such testing might have been conclusive. Indeed, a negative result would certainly be conclusive. A positive result could also have been conclusive. This argument was made in the Appeal of NIST’s response to a Request for Correction filed in 2007 by a group of scientists, an architect, and two 9/11 family members, which quoted the following statement from Materials Engineering, Inc.:
“When thermite reaction compounds are used to ignite a fire, they produce a characteristic burn pattern, and leave behind evidence. The compounds are rather unique in their chemical composition.... While some of these elements are consumed in the fire, many are also left behind in the residue.... The results [of Energy Dispersive Spectroscopy on minute traces of residue], coupled with visual evidence at the scene, provide absolute certainty that thermite reaction compounds were present....”
The Appeal therefore argued:
“[I]t is difficult to imagine a scenario in which a test for explosive residues would not be conclusive.... Unless NIST can explain a plausible scenario that would produce inconclusive explosive residue test results, its stated reason for not conducting such tests is wholly unpersuasive.”
NIST ignored this point in its response to the Appeal and provided no such scenario.
Summary
In summary, NIST provided woefully inadequate and erroneous explanations for the molten metal seen pouring out of WTC 2 and in the debris of all three buildings. Furthermore, NIST provided no explanation for evidence of extremely high temperatures in the WTC dust, except to deny that a coherent and credible hypothesis to explain it existed. Finally, NIST has not commented on the discovery of unreacted nano-thermite in the WTC dust.
Endnotes
[1] NIST: NCSTAR 1-5A, pp. 374–376.
[2] Jones, Steven: “Why Indeed Did the WTC Buildings Collapse Completely?” Journal of 9/11 Studies (September 2006).
[3] NIST: Questions and Answers about the NIST WTC Towers Investigation, Question #21.
[4] Jones, Steven: “Why Indeed Did the WTC Buildings Collapse Completely?” Journal of 9/11 Studies (September 2006).
[5] https://youtu.be/lDnbfXLUyI4
[6] https://youtu.be/nsw2j-3MCMg
[7] https://youtu.be/KtyrMt7GzyE
[8] https://youtu.be/wcqf5tL887o
[9] Jones, Steven (et al.): “Extremely High Temperatures during the World Trade Center Destruction,” Journal of 9/11 Studies (February 2008).
[10] Griffin, David Ray: The Mysterious Collapse of World Trade Center 7 (2009), pp. 43, 282. Griffin describes an email exchange between researcher Shane Geiger and NIST public affairs officer Gail Porter, which Geiger shared with Griffin.
[11] Harrit, Niels (et al.): “Active Thermitic Materials Discovered in Dust from the 9/11 World Trade Center Catastrophe,” Open Chemical Physics Journal (April 2009).
[12] McIlvaine, Bob et al.: "Appeal Filed with NIST, Pursuant to Earlier Request for Correction," Journal of 9/11 Studies (October 2007).


